List of active research topics
Softening neural interfaces
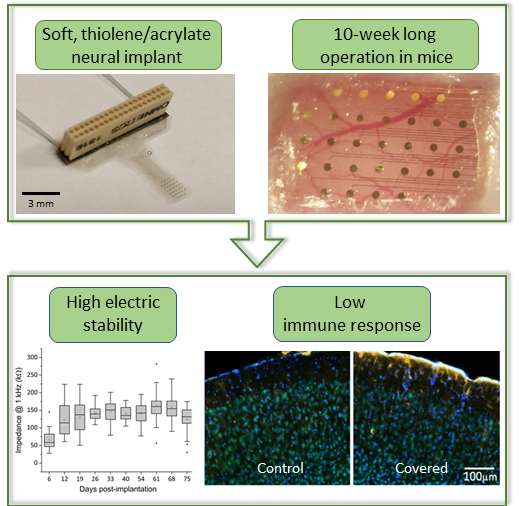
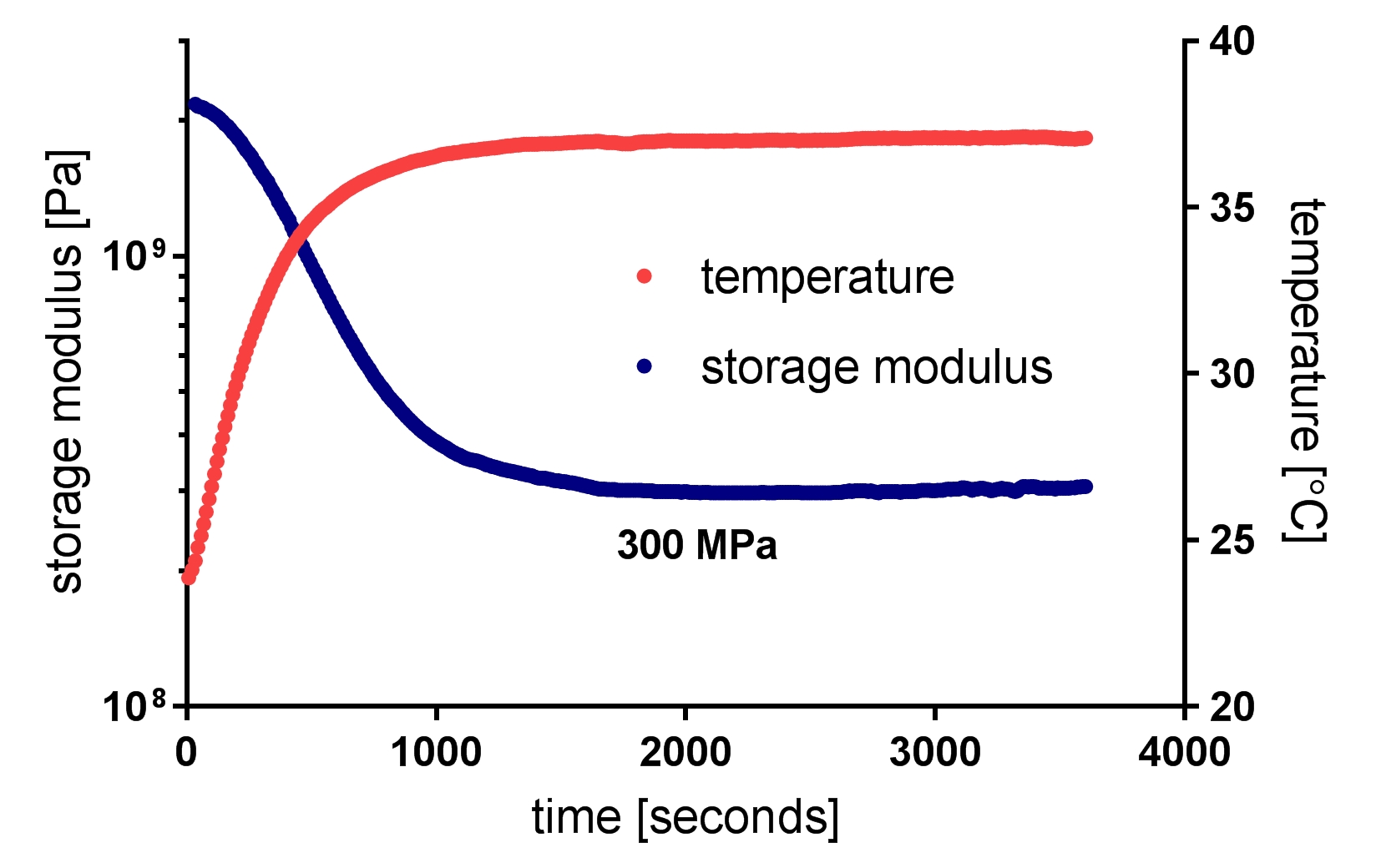
Softening neural implants that change their elastic modulus under physiological conditions are promising candidates to mitigate neuroinflammatory response due to the reduced mechanical mismatch between the artificial interface and the brain tissue. Intracortical neural probes have been used to demonstrate the viability of this material engineering approach. In our work, we present a robust technology of softening neural interfaces including laminar probes [Zatonyi, 2019] and microECoG devices [Fedor, 2021] and demonstrate their recording performance in rodent subjects in both acute and chronic conditions.
More details in the following paper:
F. Z. Fedor, M. Madarász, A. Zátonyi, Á. Szabó, T. Lőrincz, V. Danda, L. Spurgin, C. Manz, B. Rózsa, Z. Fekete, Soft, thiol-ene/acrylate based electrode array for long-term recording of intracranial EEG signals with improved biocompatibility in mice, ADVANCED MATERIALS TECHNOLOGIES (2021), DOI: 10.1002/admt.202100942
A Zátonyi, G. Orbán, R. Modi, G. Márton, D. Meszéna, I. Ulbert, A. Pongrácz, M. Ecker, W.E. Voit, A. Joshi-Imre, Z. Fekete, A softening laminar electrode for recording single unit activity from the rat hippocampus, Scientific Reports 9 (2019) 37237
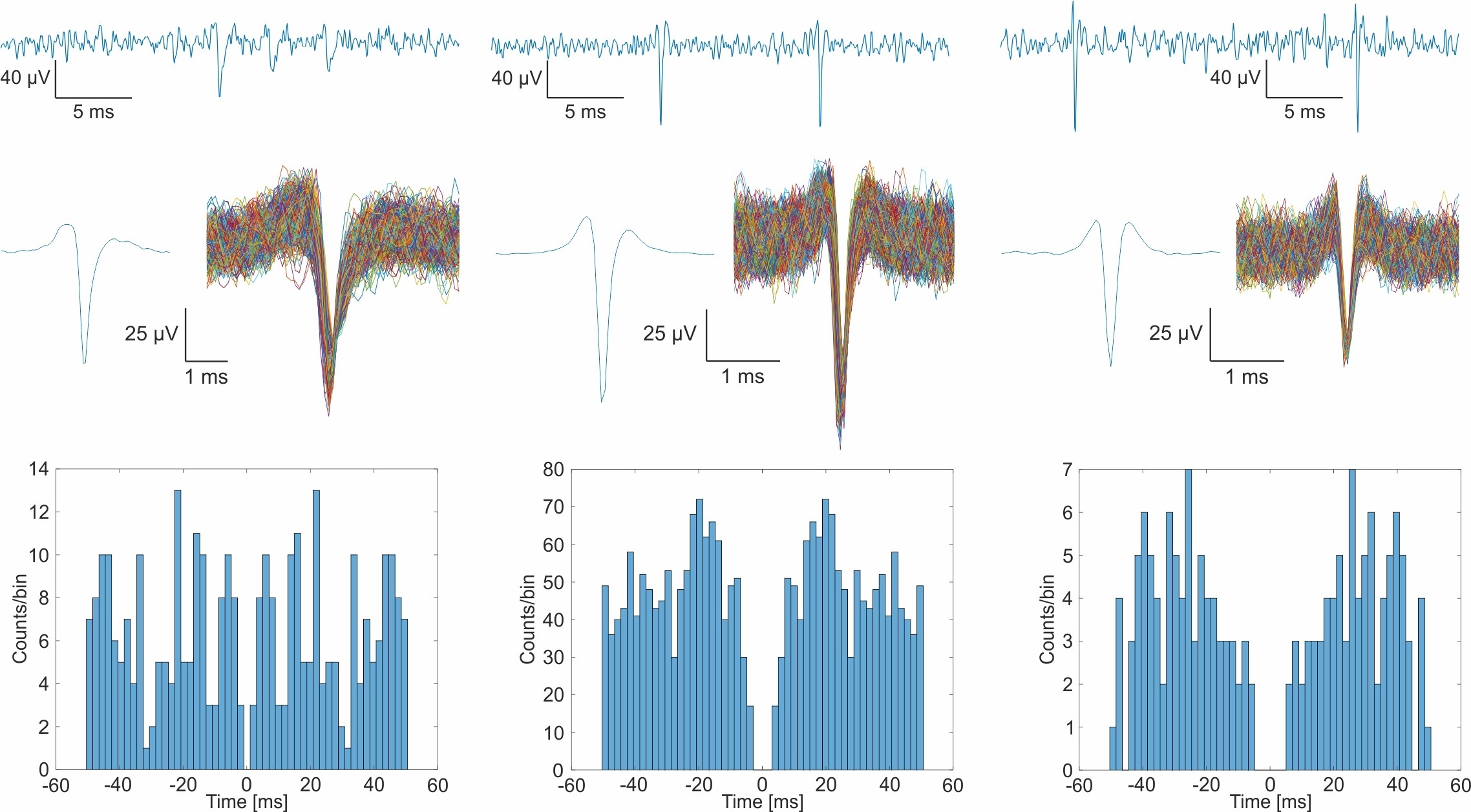
Flexible, transparent microECoGs
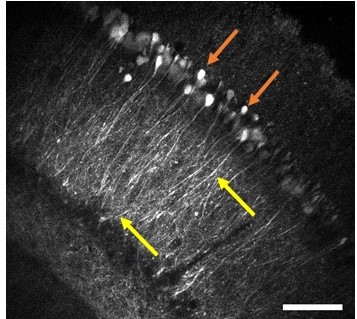
Our surface electrodes have been efficiently used in animal studies on oscillatory connectivity and were also validated in pharmacological experiments. Our recent goal is to create novel, flexible, minimally invasive microsystems that are capable of high-resolution electrical monitoring of cortical activity and can be combined with other emerging neuroimaging techniques like intrinsic optical imaging (Zatonyi, 2018], ultrafast ultrasound localization microscopy or two-photon microscopy [Zatonyi, 2020].
More details in the following papers:
A. Zátonyi, M. Madarász, Á. Szabó, T. Lőrincz, R. Hodován, B. Rózsa, Z. Fekete, Transparent, low-autofluorescence microECoG device for simultaneous Ca2+ imaging and cortical electrophysiology in vivo, Journal of Neural Engineerin 17 (2020) 016062
A. Zátonyi, F. Fedor, Zs. Borhegyi, Z. Fekete, In vitro and in vivo stability of black-platinum coatings on flexible, polymer microECoG arrays, Journal of Neural Engineering 15 (2018) 054003
A. Zátonyi, Zs. Borhegyi, M. Srivastava, D. Cserpán, Z. Somogyvári, Z Kisvárday, Z. Fekete, Functional brain mapping using optical imaging of intrinsic signals and simultaneous high-resolution cortical electrophysiology with a flexible, transparent microelectrode array, Sensors & Actuators B-Chemical 273 (2018) 519-526

Infrared neuromodulation
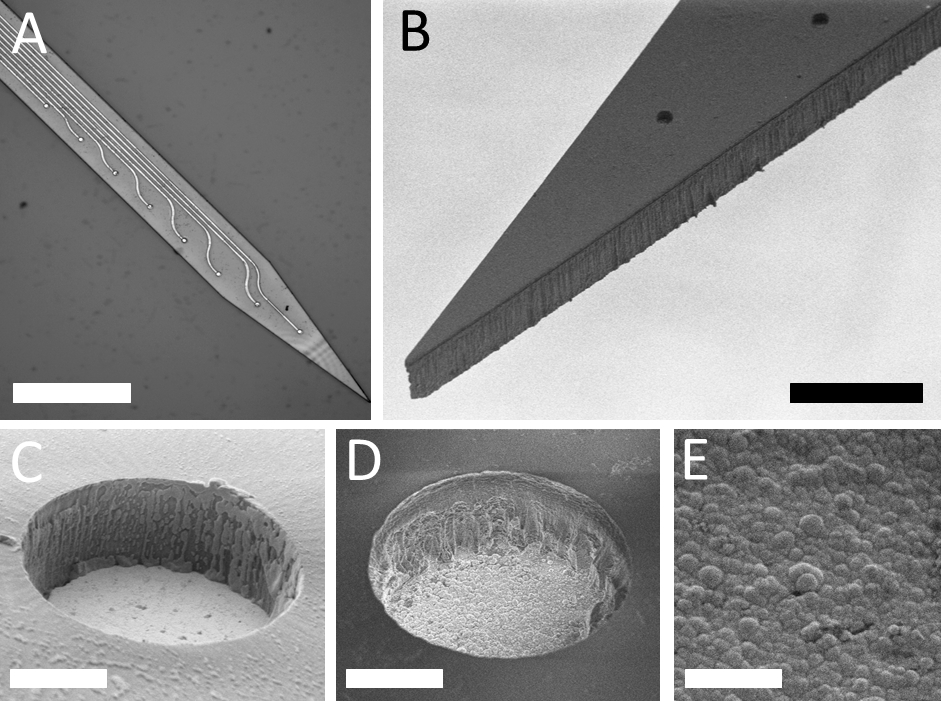
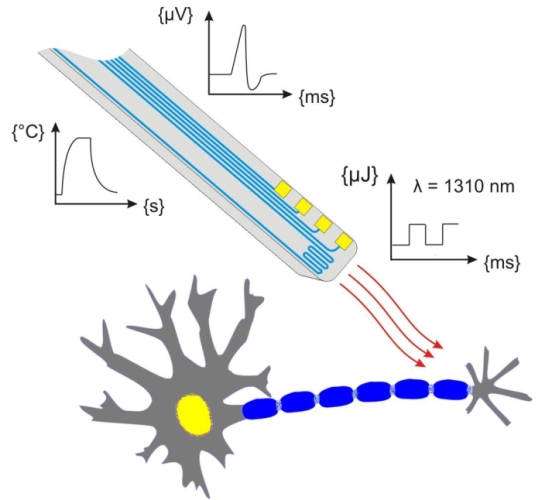
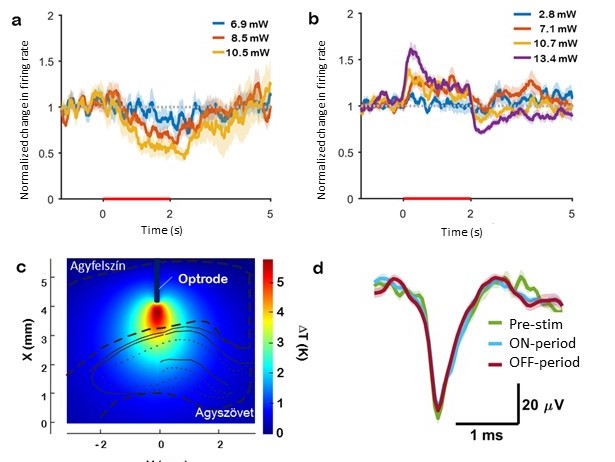
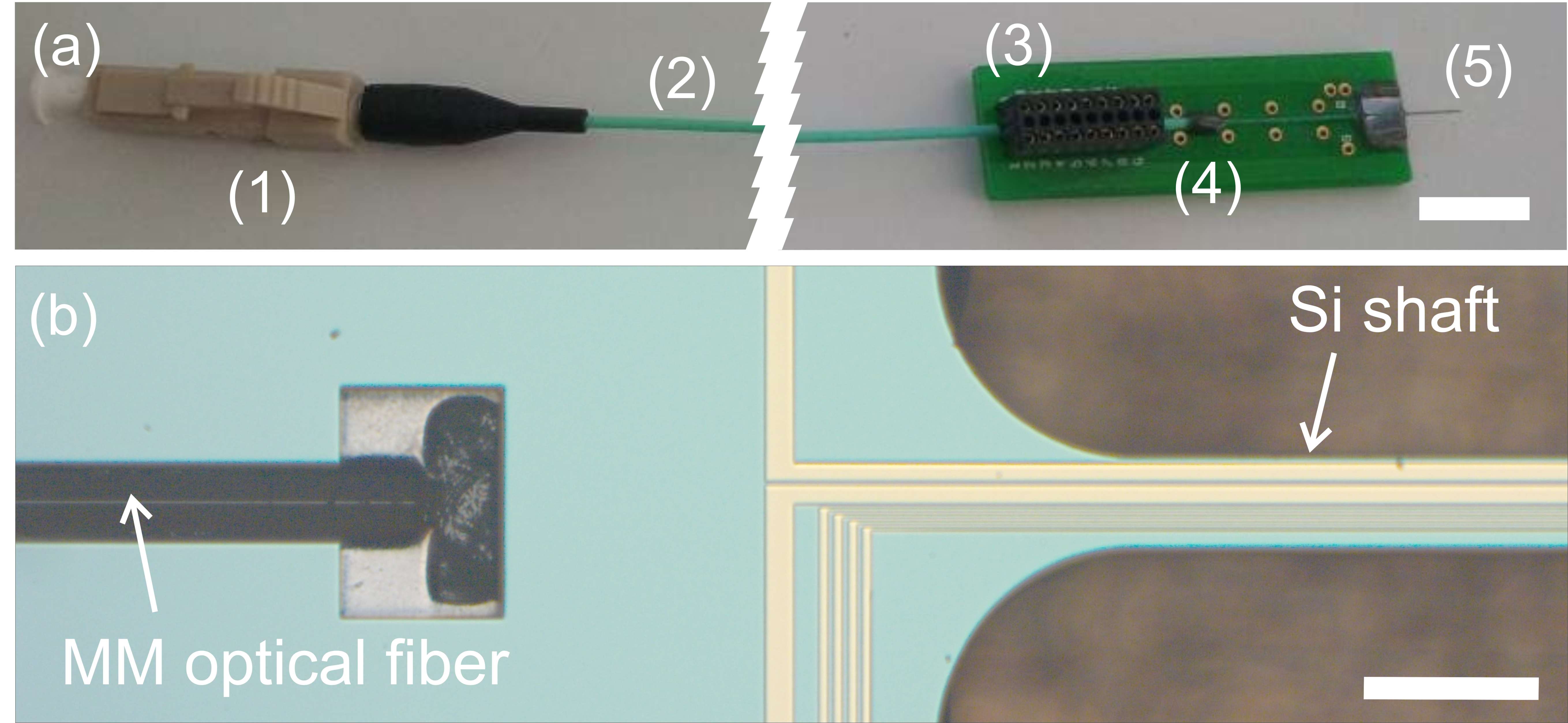
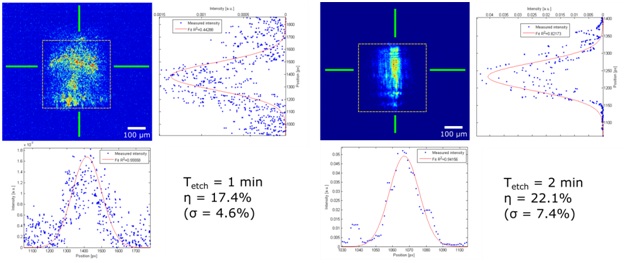
Our group proposed a novel technical approach to go beyond the current limitations of deep tissue infrared neural stimulation. We developed a penetrating neural probe capable of delivering near infrared light and simultaneously measuring background tissue temperature and action potentials. The mechanical functionality of the silicon substrate was successfully transformed to act as a waveguide due to wet chemical etching processes [Kiss, 2016] integrated into the fabrication technology of fully functional microelectrodes [Horváth, 2018]. Using this optrode device, we have demonstrated that low-energy (below 13 mW) continuous wave infrared light can be efficiently used to either excite or suppress the activity of neuronal population in the cortex of rats [Horváth, 2020].
More details in the following papers:
Á. C. Horváth, Ö. C. Boros, L. Komáromi, S. Borbély, P. Koppa, P. Barthó, Z. Fekete, Infrared neural stimulation and inhibition using an implantable silicon photonic microdevice, Microsystems & Nanoengineering 6 (2020) 44
ÁC Horváth, ÖC Boros, Ö Sepsi, S Beleznai, P Koppa, Z Fekete: A multimodal microtool for spatially controlled infrared neural stimulation in the deep brain tissue, Sensors & Actuators B-Chemical 263 (2018) 77-86
M. Kiss, P. Földesy, Z. Fekete: Optimization of a Michigan-type silicon microprobe for infrared neural stimulation, Sensors & Actuators B-Chemical 224 (2016) 676-682
Multimodal implants to characterize & suppress epilepsy
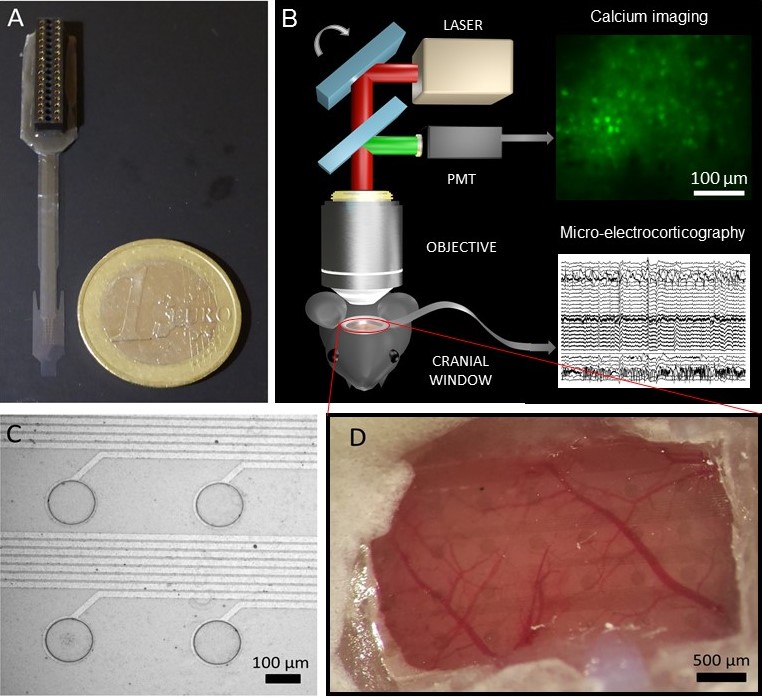
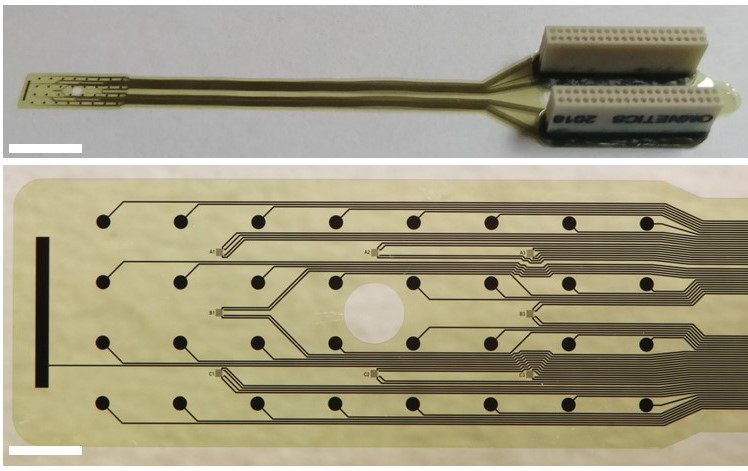
Local cooling of the brain as a therapeutic intervention is a promising alternative for patients with epilepsy who do not respond to medication. In our work, focal brain cooling in a bicuculline induced epilepsy model in rats is demonstrated and evaluated using a multimodal micro-electrocorticography (microECoG) device. We designed and experimentally tested a novel polyimide-based sensor array capable of recording microECoG and temperature signals concurrently from the cortical surface of rats. The effect of cortical cooling after seizure onset was evaluated using 32 electrophysiological sites and 8 temperature sensing elements covering the brain hemisphere, where injection of the epileptic drug was performed. The focal cooling of the cortex right above the injection site was accomplished using a miniaturized Peltier chip combined with a heat pipe to transfer heat.
More details in the following papers:
B. Csernyus, Á. Szabó, R. Fiáth, A. Zátonyi, Cs. Lázár, A. Pongrácz, Z. Fekete, A multimodal, implantable sensor array and measurement system to investigate the suppression of focal epileptic seizure using hypothermia, JOURNAL OF NEURAL ENGINEERING 18 (2021) 0460c3
B. Csernyus, Á. Szabó, A. Zátonyi, R. Hodován, Cs. Lázár, Z. Fekete*, L. Erőss, A. Pongrácz, Recent antiepileptic and neuroprotective approaches of brain cooling, SEIZURE: EUROPEAN JOURNAL OF EPILEPSY 82 (2020) 80-90
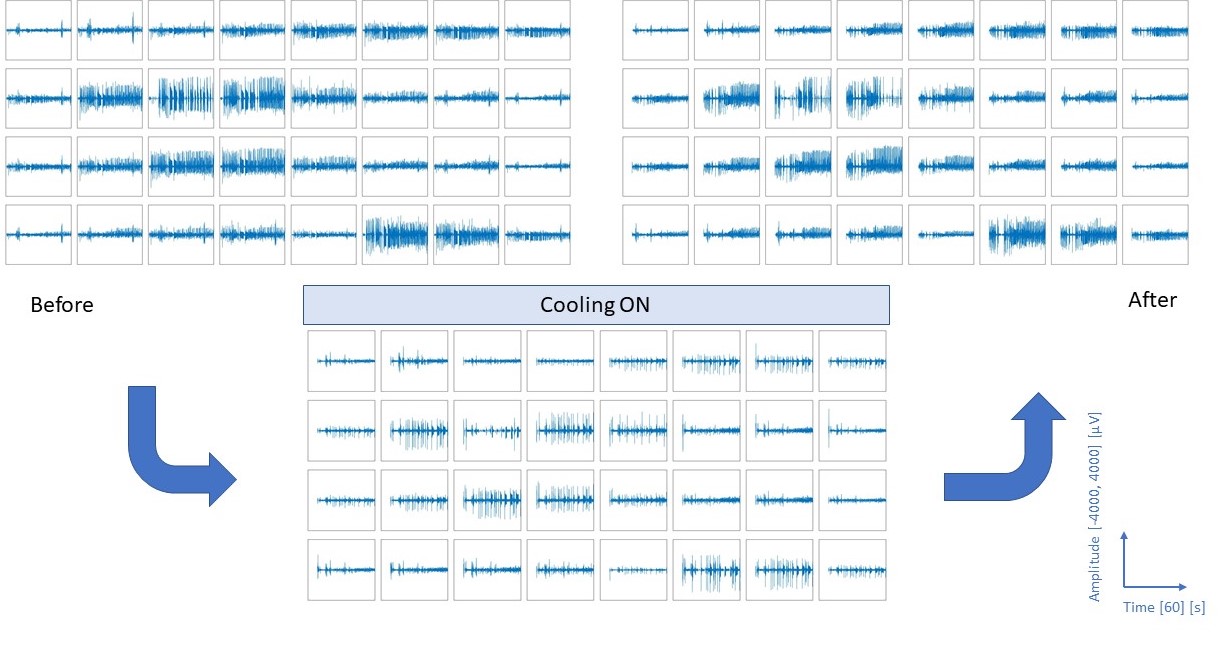
Sensors to monitor intracortical brain temperature
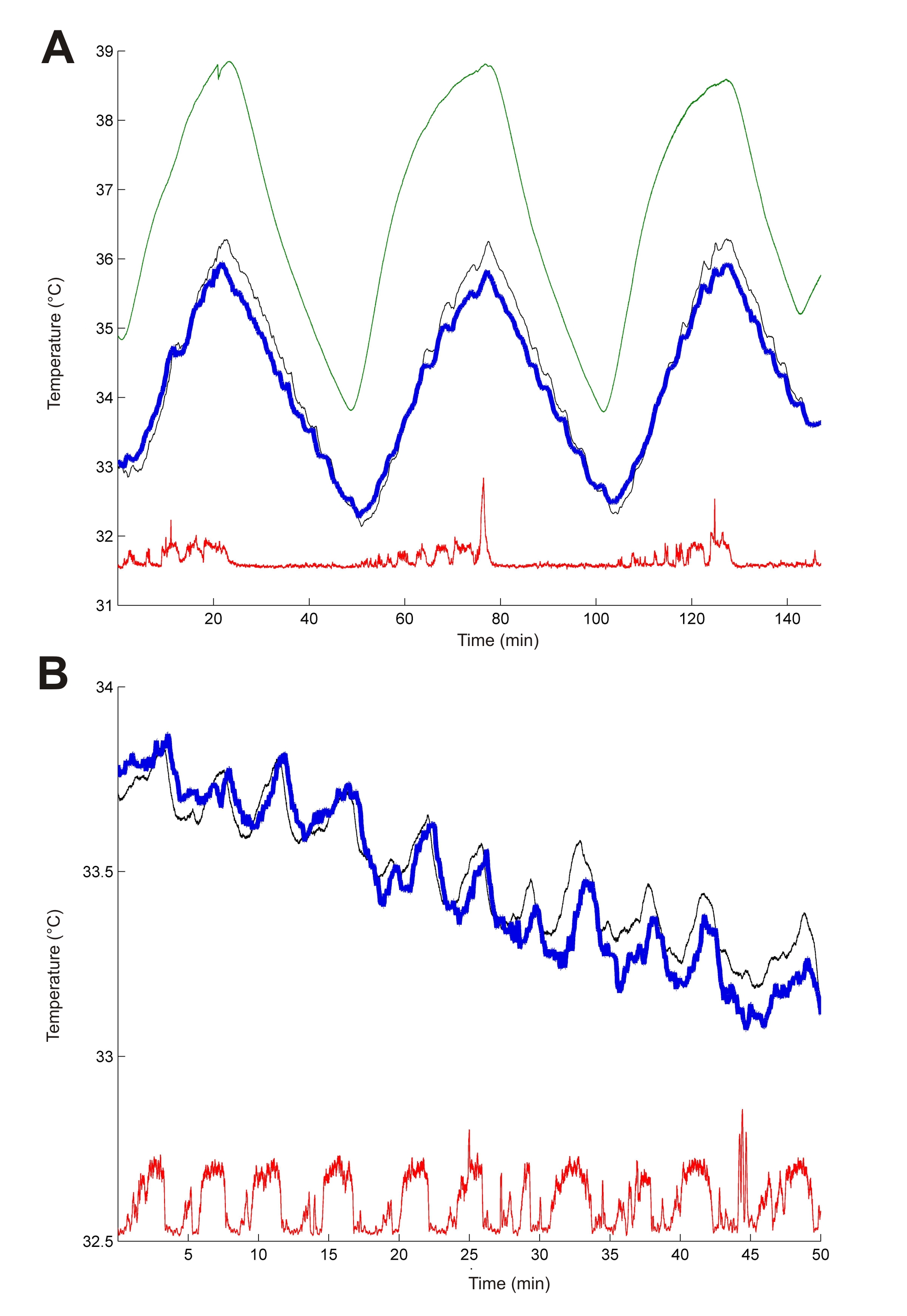
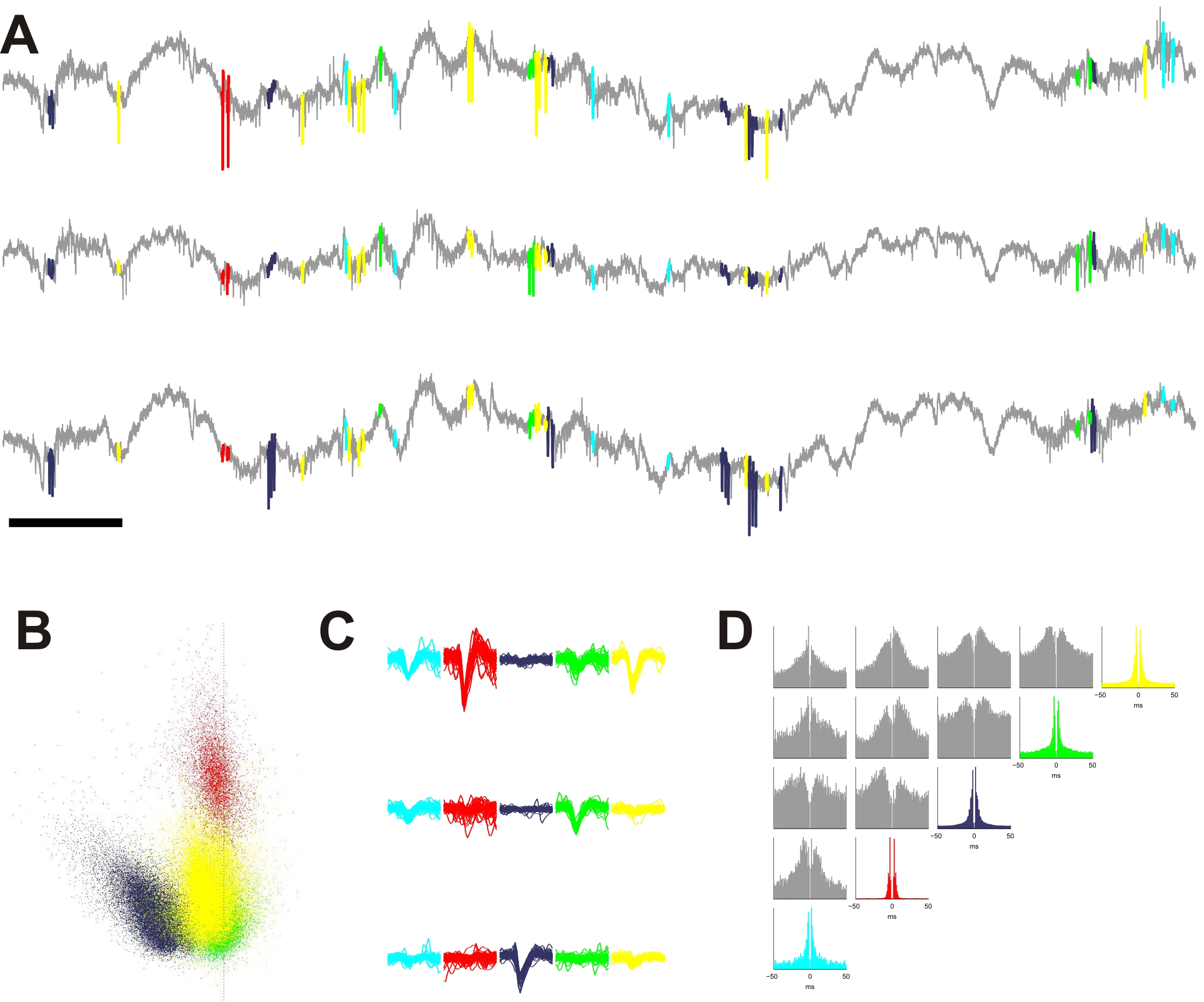
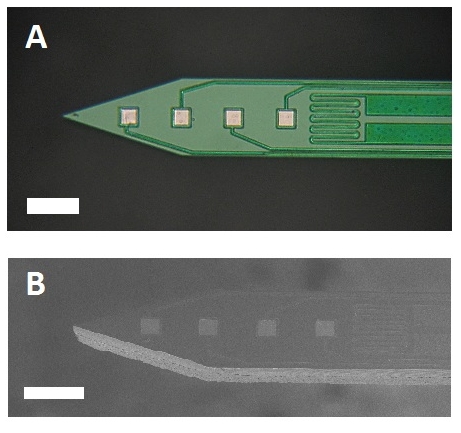
In our work, we propose an implantable, calibrated multimodal biosensor that facilitates the complex investigation of thermal changes in both cortical and deep brain regions, which records multiunit activity of neuronal populations in mice. The fabricated neural probe contains four electrical recording sites and a platinum temperature sensor filament integrated on the same probe shaft within a distance of 30 µm from the closest recording site. The feasibility of the simultaneous functionality is presented in in vivo studies. The probe was tested in the thalamus of anesthetized mice while manipulating the core temperature of the animals.
We obtained multiunit and local field recordings along with measurement of local brain temperature with accuracy of 0.14 °C. Brain temperature generally followed core body temperature, but also showed superimposed fluctuations corresponding to epochs of increased local neural activity. With the application of higher currents, we increased the local temperature by several degrees without observable tissue damage between 34-39 °C.
More details in the following paper:
M. Csernai, S. Borbély, K. Kocsis, D. Burka, Z Fekete, V. Balogh, S Káli, Z Emri, P. Barthó, Dynamics of sleep oscillations is coupled to brain temperature on multiple scales, THE JOURNAL OF NEUROPHYSIOLOGY 597 (2019) 4069-4086
Fekete, M. Csernai, K. Kocsis, Á. Cs. Horváth, A. Pongrácz, P. Barthó, Simultaneous in vivo recording of local brain temperature and electrophysiological signals with a novel neural probe, Journal of Neural Engineering 14 (2017) 034001
Drug delivery microelectrodes
We have developed a silicon based miccroelectrode for simultaneous recording of cellular electrical activity and local drug delivery Fabrication scheme relies on the smart combination of Buried Channel Technology and Etching-Before-Grinding. Our micromachining concept provides injection, sampling and electrical recording — all integrated monolithically in a long and subsequently thinned silicon microelectrode. Feasibility of our microelectrode configuration has been demostrated in in vivo experiments using either external pressure (Pongrácz et al) or iontophoretic injection (Fekete et al).
More details in the following papers:
Fekete. E. Pálfi, G. Márton, M. Handbauer, Zs. Bérces, I. Ulbert, A. Pongrácz, L. Négyessy, Combined in vivo recording of neural signals and iontophoretic injection of pathway tracers using a hollow silicon microelectrode, Sensors & Actuators B-Chemical 236 (2016), 815-824
Fekete, Technology of ultralong deep brain fluidic microelectrodes combined with etching-before-grinding, Microsystem Technologies 21 (2015) 341-344
Pongrácz, Z. Fekete, G. Márton, Zs. Bérces, I. Ulbert, P. Fürjes, Deep-brain silicon multielectrodes for simultaneous in vivo neural recording and drug delivery, Sensors and Actuators B: Chemical 189 (2013) 97–105
Z. Fekete, A. Pongrácz, P. Fürjes, G. Battistig, Improved process flow for buried channel fabrication in silicon, Microsystem Technologies 18 (2012) 353-358
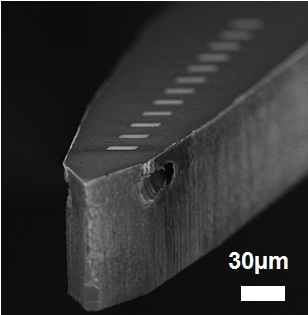
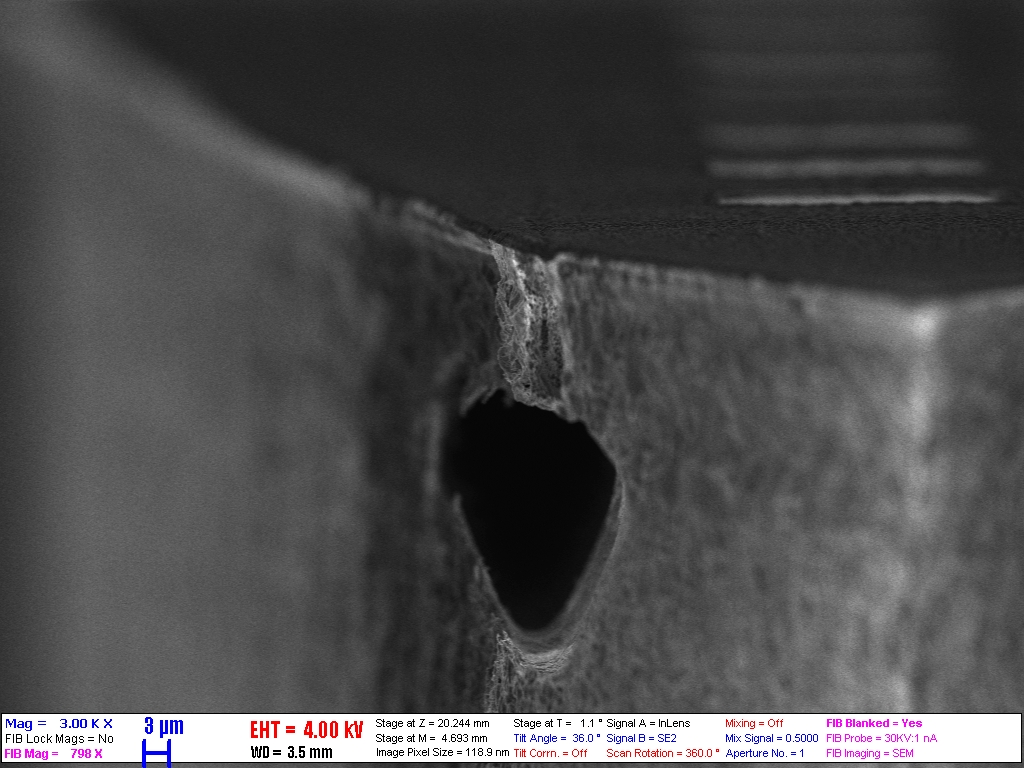
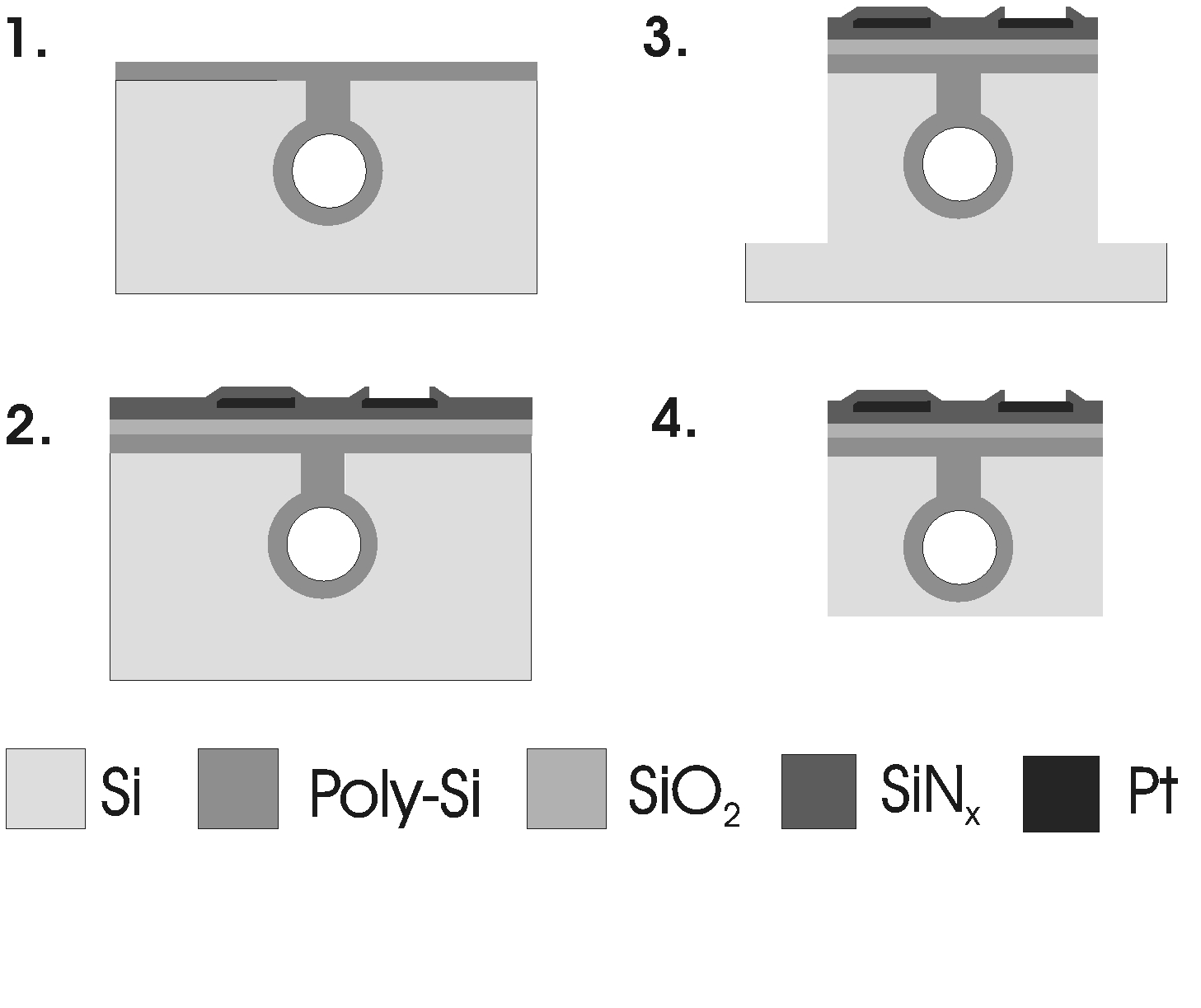
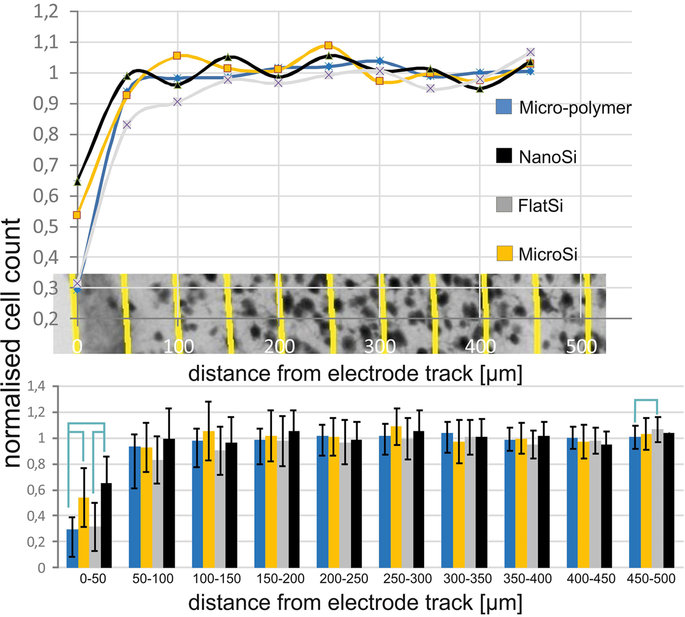
Interactions between neural cells and nanostructured interfaces
Nanostructuring the surface of neural interfaces are a promising approach to enhance cellular adhesion, biocompatibility and mitigate inflammatory response in the brain tissue. In collaboration with several neurobiology group, we have investigated the performance of several micro- and nanoengineered implant surfaces both in vitro and in vivo.
Publications:
Á. Szabó, Hanna Liliom, Z. Fekete, K. Schlett, A. Pongrácz, SU-8 microstructures alter the attachment and growth of glial cells in vitro, MATERIALS TODAY COMMUNICATIONS 27 (2021) 102336
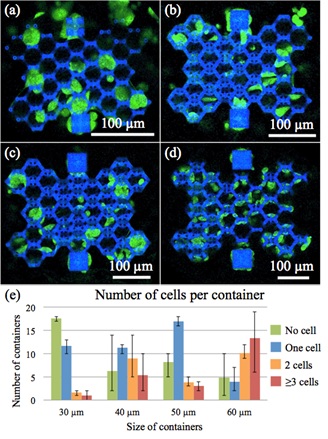
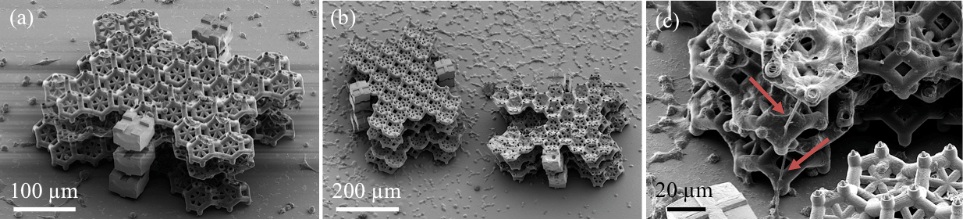
F. Larramendy, S. Yoshida, D. Maier, Z. Fekete*, S. Takeuchi, O. Paul, 3D arrays of microcages by two-photon lithography by spatial organization of living cells, LAB ON A CHIP 19 (2019) 875-884, IF: 6.774
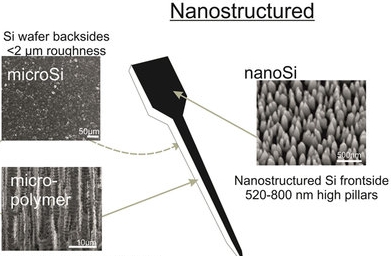
H. Liliom, P. Lajer, Zs. Bérces, B. Csernyus, Á. Szabó, D. Pinke, P. Lőw, Z. Fekete, A. Pongrácz, K. Schlett, Comparing the effects of uncoated nanostructured surfaces on primary neurons and astrocytes, JOURNAL OF BIOMEDICAL MATERIALS RESEARCH: PART A 107 (2019) 2350-2359
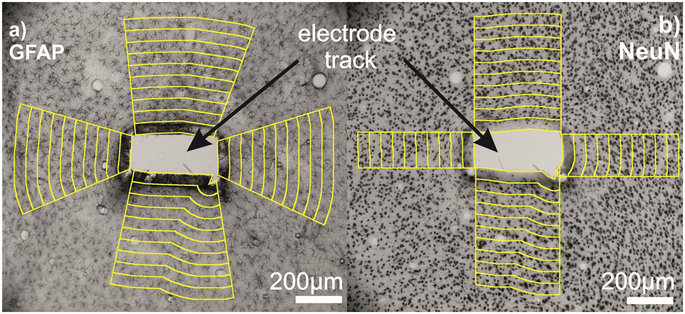
Zs. Bérces, J. Pomothy, Á. Cs. Horváth, T. Kőhidi, É. Benyei, Z. Fekete*, E. Madarász, A. Pongrácz, Effect of nanostructures on anchoring stem cell-derived neural tissue to artificial surfaces, JOURNAL OF NEURAL ENGINEERING 15 (2018) 056030
Zs. Bérces, K. Tóth, G. Márton, I. Pál, B. Kováts-Megyesi, Z. Fekete, I. Ulbert, A. Pongracz, Neurobiochemical changes in the vicinity of a nanostructured neural implant. Scientific Reports 6 (2016) 35944
…